Managing diabetes requires constant vigilance, as fluctuations in glucose levels can lead to serious and potentially life-threatening complications. Continuous glucose monitors (CGMs) are wearable medical devices that continuously track glucose levels in the blood and transmit the data to a device or receiver to display glucose levels in real time for informed therapy to take place. CGMs are incredibly beneficial medical devices for patients with diabetes as they enable patients to quickly respond to spikes or drops in their glucose levels and administer insulin accordingly. Modern CGMs also interface with insulin pumps to automate insulin therapy to help reduce adverse health effects from delayed insulin treatment in a closed-loop system. These closed-loop systems will use the CGM to read glucose levels and send signals to an insulin pump that will then inject insulin when needed to stabilize a patient's blood level.
When designing continuous glucose monitors, medical device designers have many things to consider. Some considerations include the following:
- The need for security regarding health information and communication to mobile applications and insulin pumps
- Reliable and precise measurements to ensure that glucose readings are accurate such that alerts or insulin delivery are correct.
- The need to design around a small footprint such that the CGM is discrete and comfortable to wear
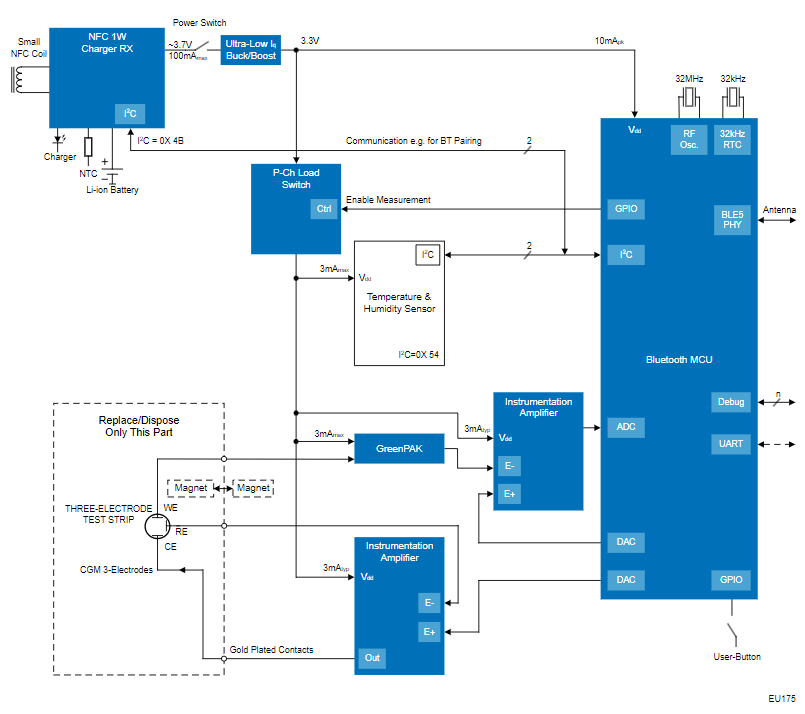
Renesas' Continuous Glucose Monitor (CGM) for Closed-Loop Operation with an Insulin Pump offers robust health information security, accurate glucose level readings, and a small footprint with vetted modules and solutions compliant with regulatory specifications that shorten time-to-market for medical device development.
Health Information Security & Time-to-Market
This system design features the RX23W microcontroller which enables a single-chip solution with security functions for communication procedures such as Bluetooth® Low-Energy (LE) 5.0 and IoT to ensure that health information from the CGM to digital health platforms (DHPs) such as mobile applications and insulin pumps remains secure. These security functions ensure patient data remains secure, preventing unauthorized access and maintaining patient privacy. Additionally, the RX23W microcontroller has obtained Radio Law (Japan), FCC/ISED (North America), and CE (Europe) certifications for radio frequency (RF) compliance which shortens time-to-market as it is compliant with specifications laid out by regulatory bodies for medical devices.
Accurate Glucose Level Readings
Glucose sensors tend to produce very small signals on the order of microvolts on the raw output. Thus in many designs, amplifiers or Analog Front-Ends (AFE) with integrated circuits for analog signal conditioning are used to boost the signal into the millivolt range so that it can be accurately digitalized and processed.
Leveraging Renesas' programmable mixed-signal GreenPAK IC with integrated signal conditioning, amplification of these small glucose sensor readings can be done with high precision and flexible gain adjustment all within a compact IC to reduce external components, lower BOM costs, and have low-power operation with its low current consumption.
Small & Modular Footprint Design
This modular system design features disposable electrodes with a convenient magnetic connection to the main electronic module powered by a Lithium-ion battery that is chargeable through wireless NFC charging. This design promotes product longevity for the end user and reduces waste by only needing to dispose of the test end, making this a more sustainable design not only for the environment but also for the patient as they only need to change out the test end rather than the entire CGM unit. Thus, this not only reduces environmental waste but also lowers the costs for patients by extending the life of the core device.
Components of this CGM system design boast small footprints that can fit into a condensed form factor for a discrete and comfortable-to-wear medical device. The RX23W microcontroller offers the industry's smallest footprint for a module, a PTX30W NFC charger offers a single-chip solution for NFC and wireless charging protocols, and a combination of the ISL28533 instrumentation amplifier with the SLG47004 Programmable Mixed-Signal Matrix AnalogPAK enables robust signal conditioning for precise glucose readings while retaining a small footprint and form factor.
In conclusion, the Renesas Continuous Glucose Monitor (CGM) for Closed-Loop Operation offers a highly integrated solution that addresses the key challenges in diabetes management. By providing accurate glucose readings, this system ensures precise insulin delivery, significantly reducing the risk of complications associated with fluctuating glucose levels. Its compact and modular design not only enhances patient comfort but also extends the device's lifespan, making it a cost-effective and environmentally friendly option. Moreover, robust health information security and compliance with global regulatory standards facilitate a faster time-to-market, empowering medical device manufacturers to deliver innovative and reliable solutions to patients quickly.
To learn more, see our Continuous Glucose Monitoring (CGM) for Closed-Loop Operation with Insulin Pump winning combination.
Also, take a look at our Insulin Pump with Closed-Loop Operation for Continuous Glucose Monitoring (CGM) winning combination.
